HungHau, News & Event
REGULATION OF EXPORTING AGRICULTURAL PRODUCTS TO THE UNITED STATES MARKET
Recently, representatives of HungHau Foods company attended to the seminar “Requests of Foods & Pharmaceutical management agencies in Exporting Foods and Beverages to the United states”.
At the seminar, there was Mr. David Lennarz, president – co-founder of Register Corp, who lectured on FDA regulations regarding foods, medicine, medical devices and cosmetics.

During the seminar, Mr. David has presented about the rules to ensure Vietnam’s food exports to the US market are safe and qualified. The Foods and Drug Administration (FDA) is implementing several new provisions of the law on modernization of food safety.
- Registered production facilities:
Exporting foods to the US market. All food processing establishments for export of Vietnam and some countries that have regulations on food export to the US market must conduct a new registration, or registration again at the U.S. Food and Drug Administration (FDA) including facilities manufacturing, processing, packaging ỏ storing goods …. So, only establishments registered with the FDA will receive the business code to be allowed to export to the United States. All exported products will be strictly inspected at the custom, if there are any case of ineligible, the goods will be refused and keep at the border gates. Normally, establishments will have to register again every two years to receive a new business code.
- Register for a representative in the US.
Exporting foods to the US market. Along with being granted a new business code, exporters must register an additional US representative according to FDA standards for their facility. Exporting foods to the US market, the representative may be an individual, a company or an organization based in the US, they act as the main liaison with the FDA and are committed to answering any questions or inquiries of FDA, which related to export facilities or exports.
Response time is 24 hours after receiving the notice. The representative of the United States will also pay for all expenses related to the to the inspection of exports by the FDA on behalf of the exporter.
Exporting food to the US market. Food products include all items used to process food and drink for humans and animals, or just a part of food and drink.
Food items are collectively divided into 3 groups:
- Food and beverage group
- Functional food group
- Alcoholic beverage group
Currently, there are many agencies in the US that can help us register the export facility of FDA without paying any cost through the US Food and Drug Administration’s website or through service companies.
Also, at the seminar mentioned above, Mr. David Lennarz also presented the regulations on product labeling.
The FDA has published a new nutrition fact panel to show information scientifically, including the relationship between diet and chronic diseases such as cardiovascular disease and obesity. The new labeling will help consumers select foods more easily.
SOME KEY POINTS IN THE NEW NUTRITION TABLE
- New design
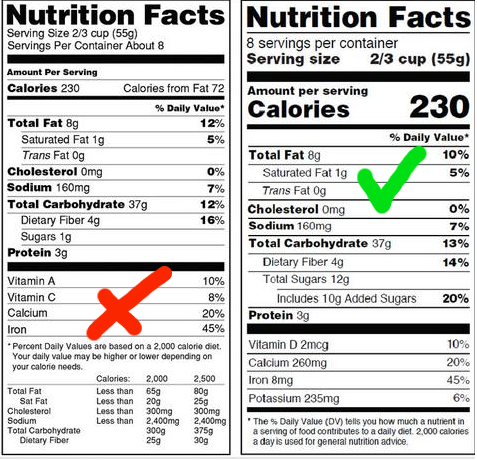
The “iconic” icons remain the same but have been updated to ensure that users can get the information they need to make decisions about what foods they consume. For example, change the size of the words “Calories”, “Servings per container” and “serving size”. The number of calories is bolded to highlight.
Manufacturers must give exact numbers for Vitamin D, Calcium, Iron, and potassium. They can declare their own vitamins and minerals. The caption on the Daily value was also changed to better explain the specific daily nutrient intake as follows. “The % Daily value tells how much a nutrient in a serving of food eating to a daily diet. 2.000 calories a day is used for general nutrition advice.”
- Reflecting updated information on nutritional science
On the label will add “add sugar added” as a percentage of the daily value. The scientific figures show that it will be difficult to meet the nutritional needs while ensuring the right amount of calorie when you add 10% of calories from added sugar. This is also in line with dietary guidelines for Americans 2015-2020.
The list of required or permitted nutrient claims is updated. Vitamin D and Potassium are required to show. Calcium and iron will continue to be required. Vitamin A and C will no longer be required but can be put on voluntary basis.
“total fat, “saturated fat” and “Trans-fat” must be shown. However, “calories from fat” is removed because studies show that type of fat is more important than quantity.
Daily nutritional values such as sodium, fiber, and vitamin D are being updated based on scientific evidence from the institute of Medicine, and other reports such as the 2015 report of the Dietary Guidelines Committee used in the development of dietary guidelines for Americans 2015-2020.
- Updates to size, and nutrition labeling
By law, sizes must be based on the amount of food or drink that people are consuming, not what they should consume. The amount of food consumed has changed size the previous serving size was established in 1993. For example, the ice cream ration set to ½ cup has change to 2/3. Soda consumptions also changed from 8 ounces to 12 ounces.
Packing size will affect what we consume. Therefore, packages that can be used 1 to 2 times such as 20-ounce soda cans or 14-ounce soup boxes, calories and other nutrients will be required to be labeled once used because they are often used only once.
For products with more than one serving, manufacturers will provide a “dual column” to indicate calories and nutrition for both “per serving” and “per packing” or “per unit”. For example, a 24-ounce or half-liter soda bottle. With 2 separate labels, people can easily know how much nutrients and calories are consumed if they eat the whole package one time.
Format of old and new nutrition ingredient labels:
Application time:
Effective day: regulationson nutrition ingredient labels are effective from 26th July 2016.
Compliance date:
1st January 2020 for producers with more than $10 million in annual sales
1st January 2021 for producer with less that $10 million in annual sales.
